Ulcerations
Ulcerations are open sores that can be caused by underlying conditions such as cancer or diabetes. Ulcers are the most common type of chronic wounds. When surface cells die off and break, skin ulcers occur.
Neuropathic ulcer in a 95-year-old female
Patient History: Arthritis, Asthma, Cancer, Coronary Artery Disease, DM11, Hypertension, Other heart disease, Vascular disease
Treatment: 9 artacent applications across 11 weeks
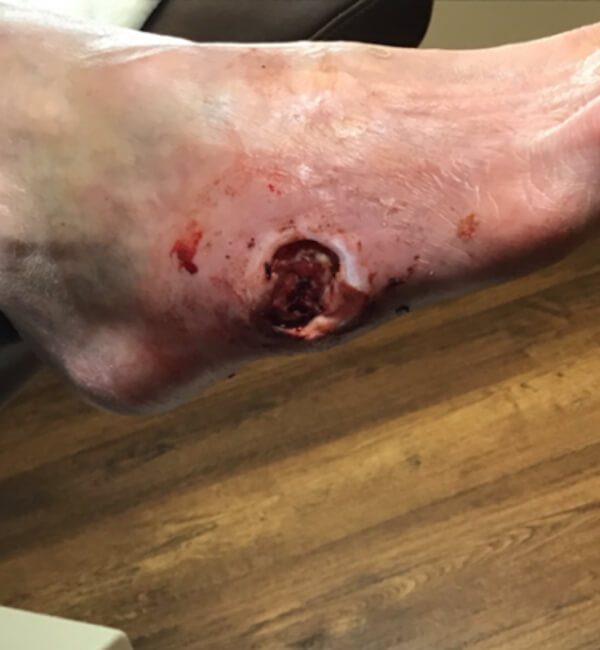
Artacent® Week 1
Wound Size: 1.3 x 0.8 x 0.7 cm
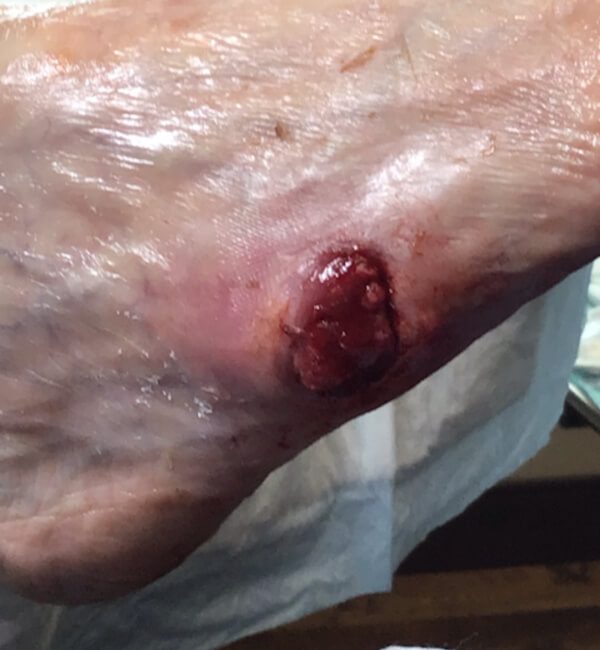
Artacent® Week 7:
Wound Size: 1.7cm length x 1.8cm width x 0.1cm depth
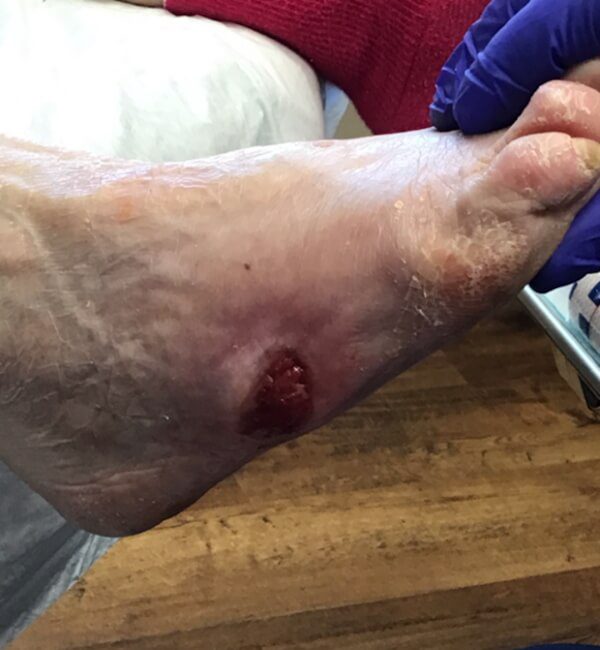
Artacent® Week 8:
Wound Size: 1.7cm length x 1.7cm width x 0.1cm depth
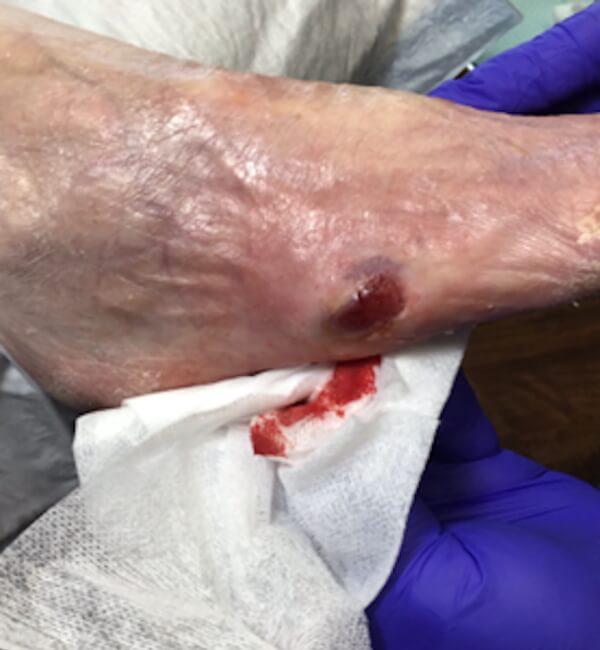
Artacent® Week 9:
Wound Size: 1.7 cm length x 1.7cm width x 0.1cm depth
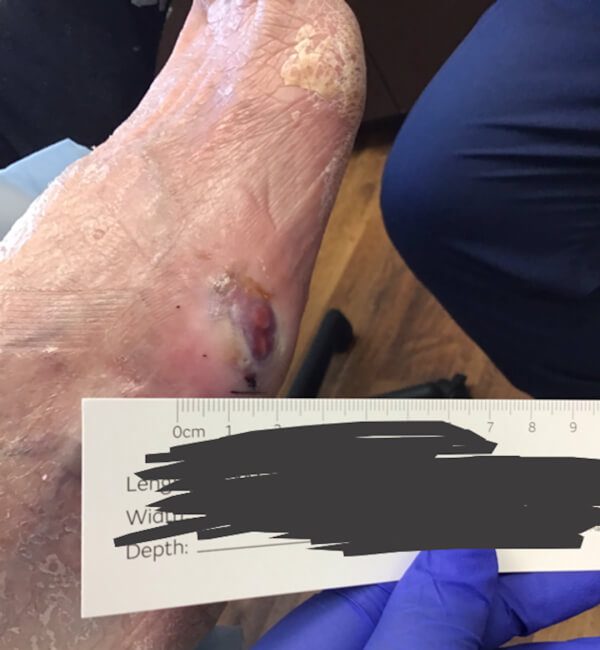
Artacent® Week 10:
Wound Size: 1.2cm length x 0.9cm width x 0.1cm depth
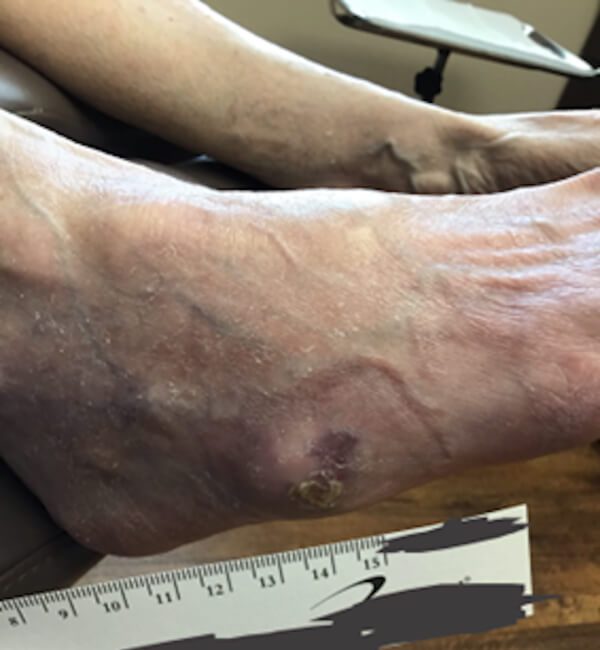
Post treatment closure
Extensive necrotic ulcerations in a 72-year-old man
Treatment: 9 artacent applications across 11 weeks
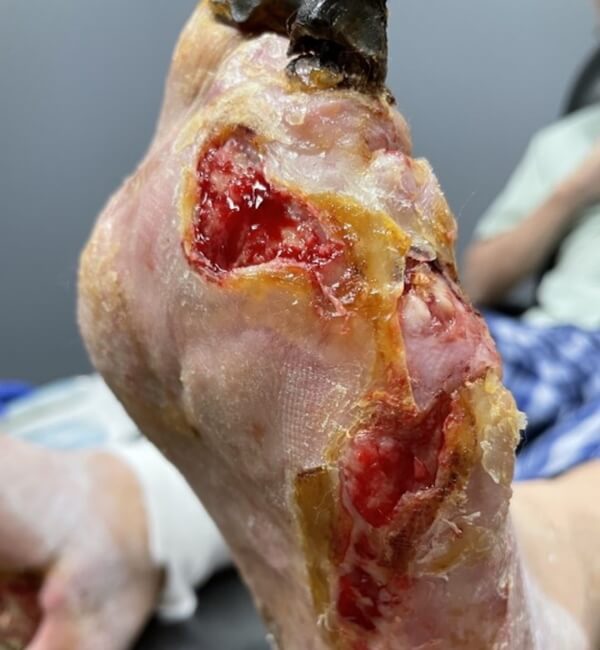
Artacent® Week 1:
Wound Size: 8.5 length cm x 5 width cm x 0.1 depth cm
Wound Area: 42.5 cm²
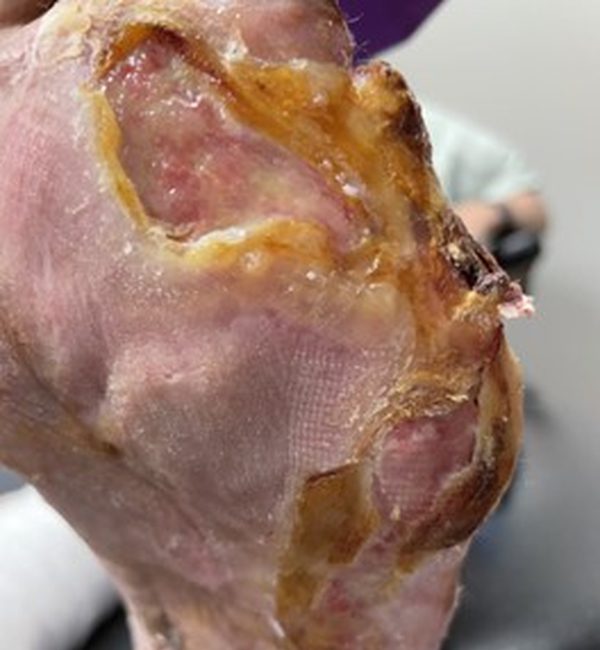
Artacent® Week 2:
Wound Size: 8.4 length cm x 4.2 width cm x0.1 depth cm
Wound Area: 35.28 cm²
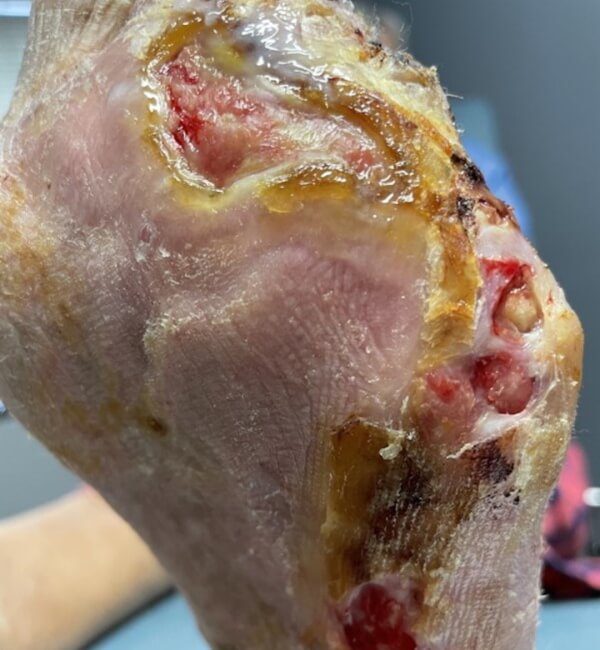
Artacent® Week 4:
Wound Size: 8.1 length cm x 4.3 width cm x 0.1 depth cm
Wound Area: 34.83 cm²
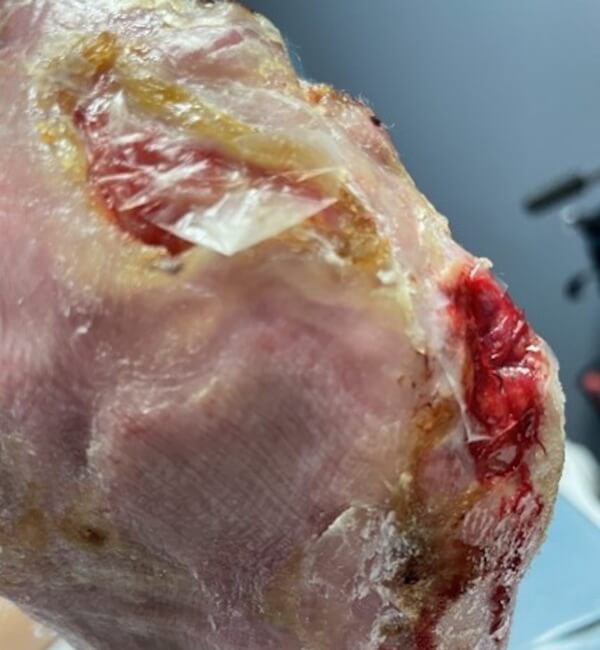
Artacent® Week 6:
Wound Size: 8.5 length cm x 4.7 width cm x 0.1 depth cm
Wound Area: 39.95 cm²
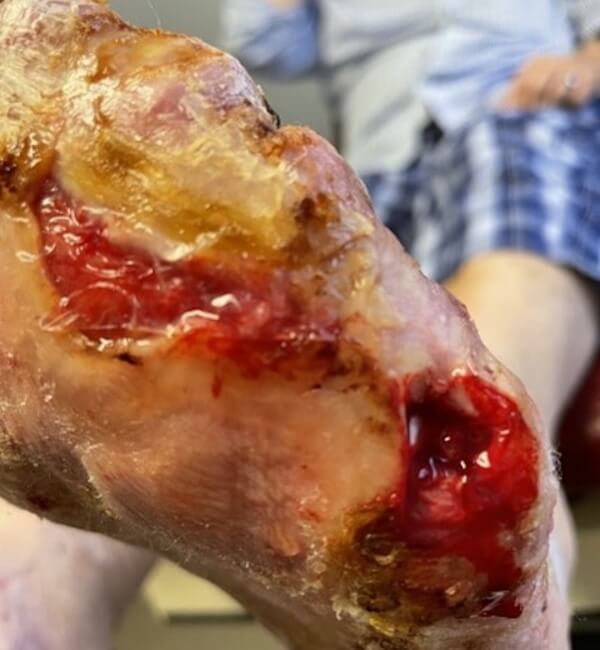
Artacent® Week 9:
Wound Size: 2.6 length cm x 4 width cm x 0.1 depth cm
Wound Area: 10.4 cm²
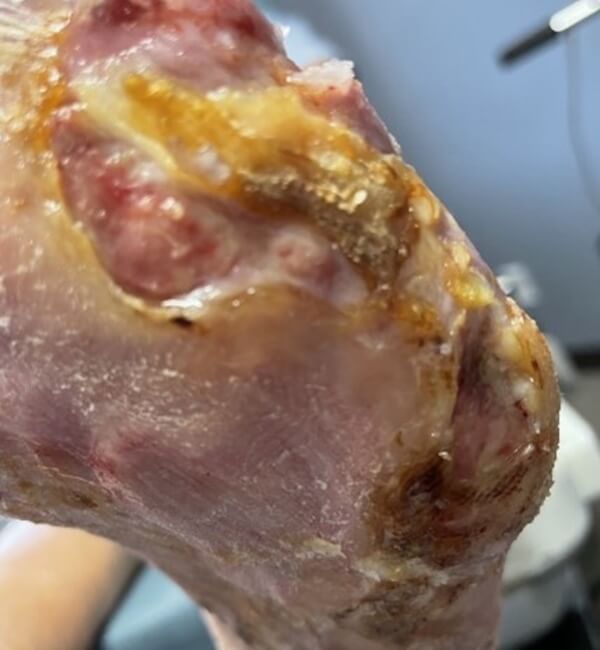
Artacent® Week 10:
Wound Size: 2.5 length cm x 3.6 width cm x 0.1 depth cm
Wound Area: 9.0 cm²
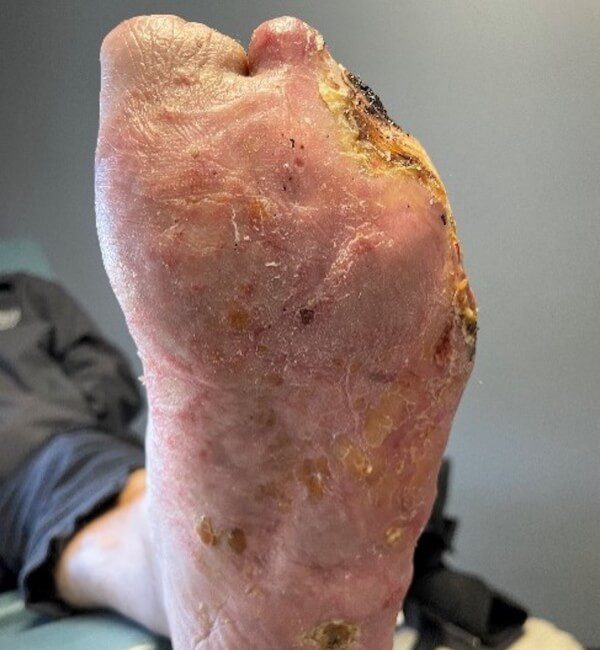
Post treatment closure
Mixed arterial wound presented in 75-year-old male
Patient History: HTN, HLD, CAD
Treatment: 11 artacent applications, complete closure at week 16
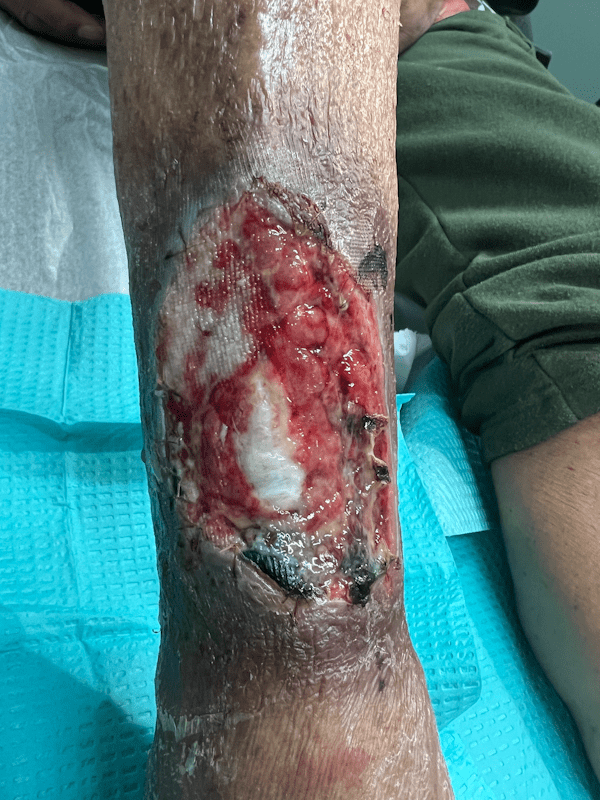
Artacent Wound® Week 1:
Wound Size: 8.5x5x0.1cm
Wound Area: 42.5 cm²
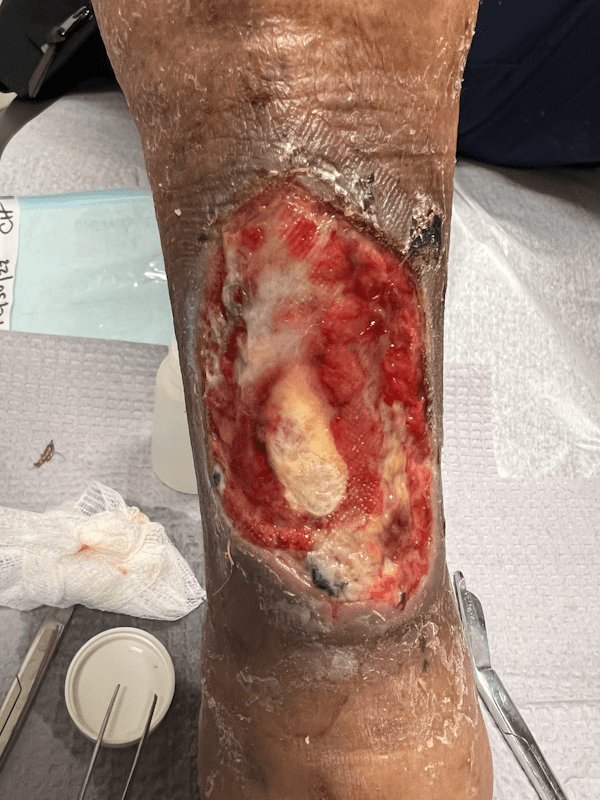
Artacent Wound® Week 2:
Wound Size: 8.4 x 4.2 x 0.1cm
Wound Area: 35.28 cm²
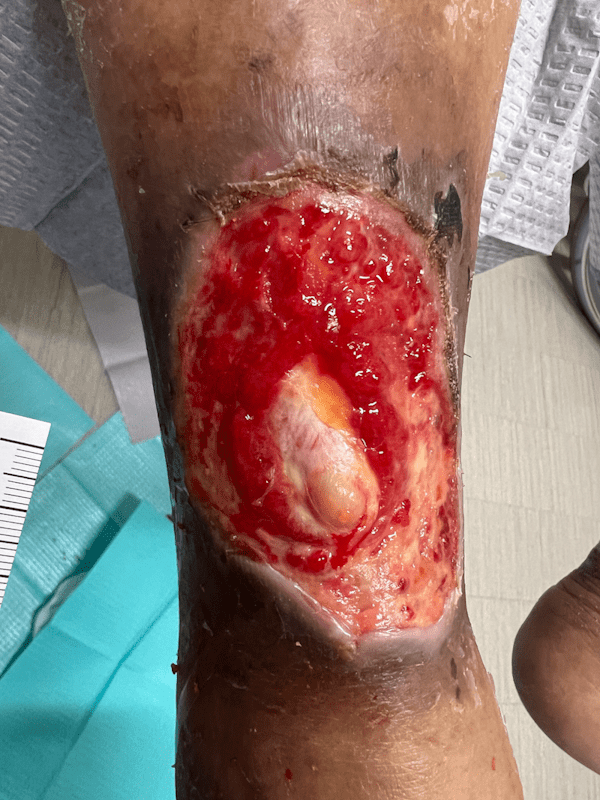
Artacent Wound® Week 3:
Wound Size: 7.6 x 4.5 x 0.1cm
Wound Area: 34.2 cm²
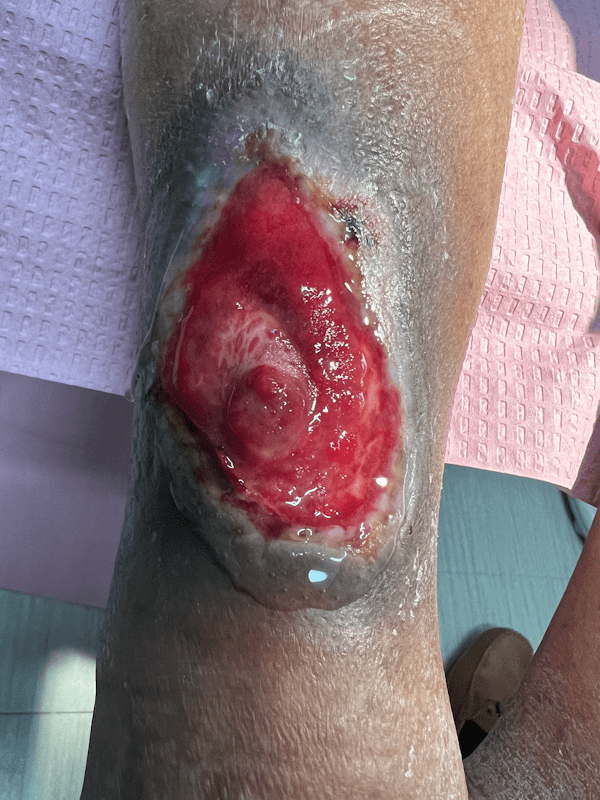
Artacent Wound® Week 4:
Wound Size: 7.6 x 4.3 x 0.1cm
Wound Area: 32.68 cm²
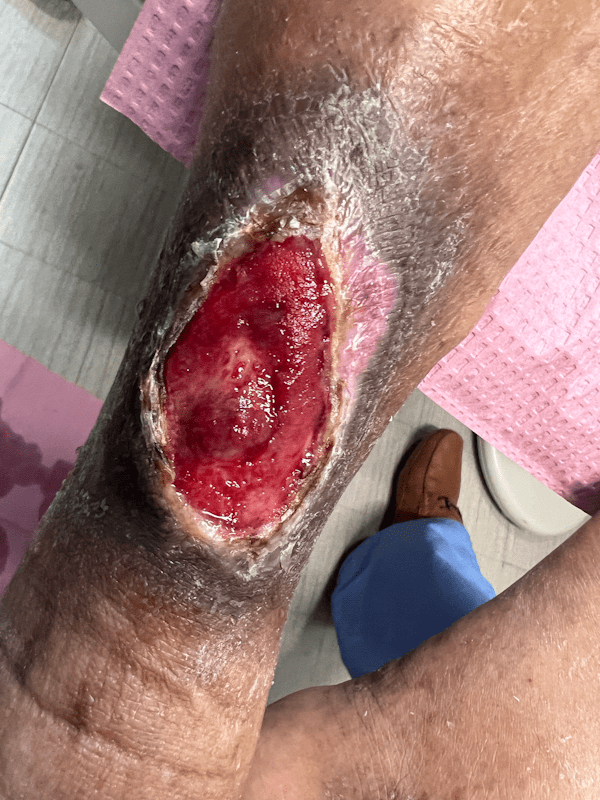
Artacent Wound® Week 5:
Wound Size: 7.4 x 4.3 x 0.1cm
Wound Area: 31.82 cm²
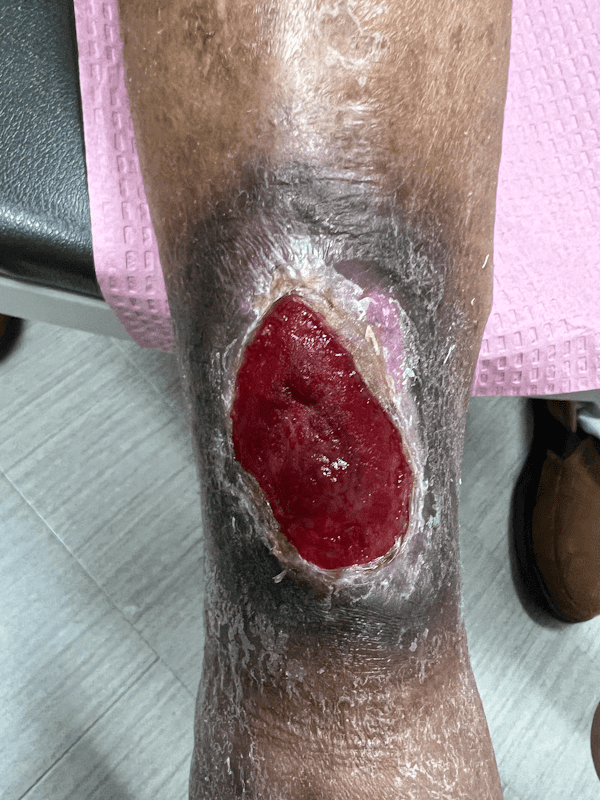
Artacent Wound® Week 6:
Wound Size: 7.3 x 4.3 x 0.1cm
Wound Area: 31.39 cm²
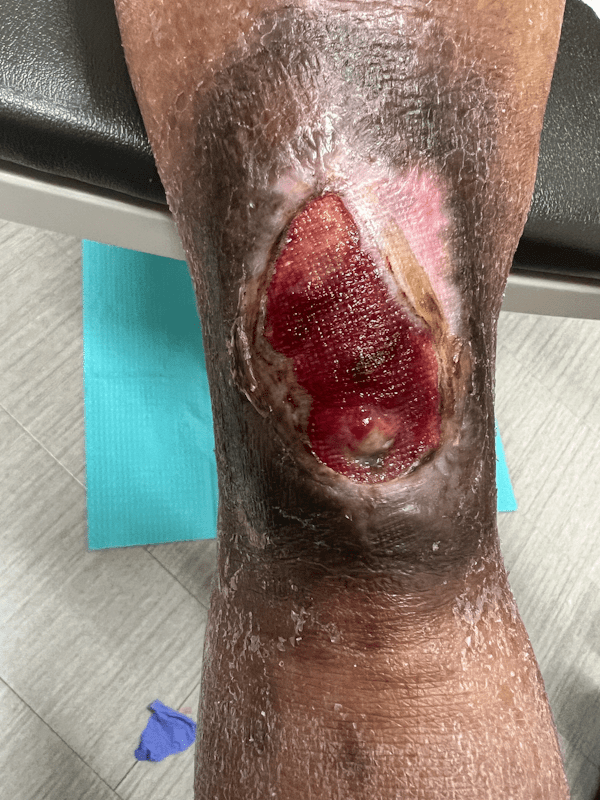
Artacent Wound® Week 7:
Wound Size: 6.8 x 3.8 x 0.1cm
Wound Area: 25.84 cm²
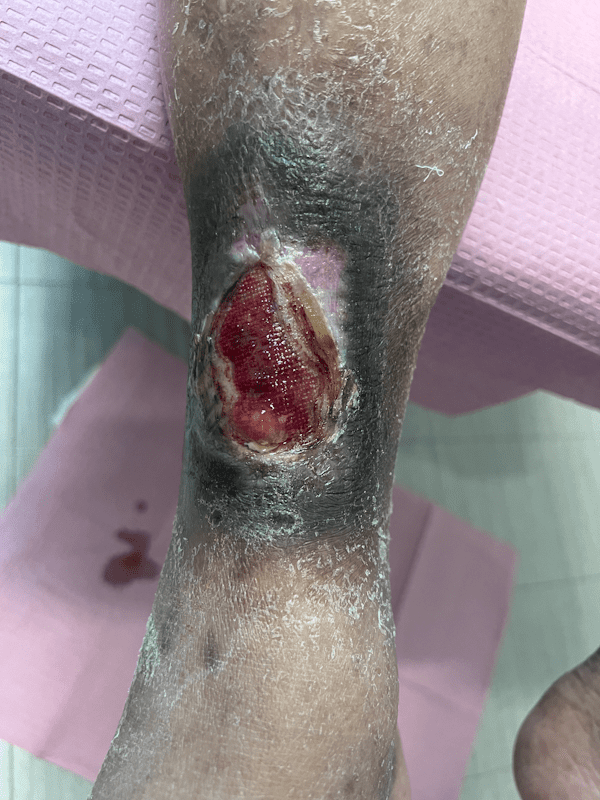
Artacent Wound® Week 8:
Wound Size: 6.1 x 3.2 x 0.1cm. Artacent applied
Wound Area: 19.52 cm²
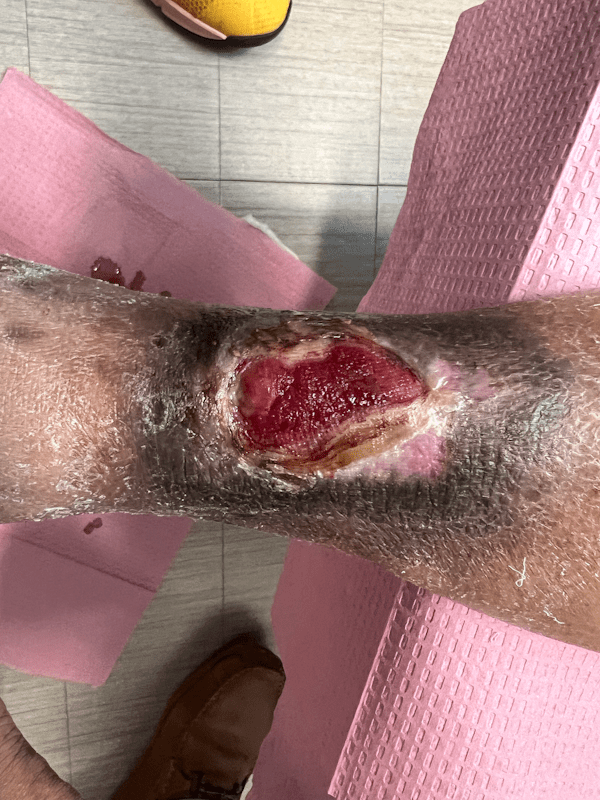
Artacent Wound® Week 9:
Wound Size: 6.0 x 3.2 x 0.1cm
Wound Area: 19.2 cm²
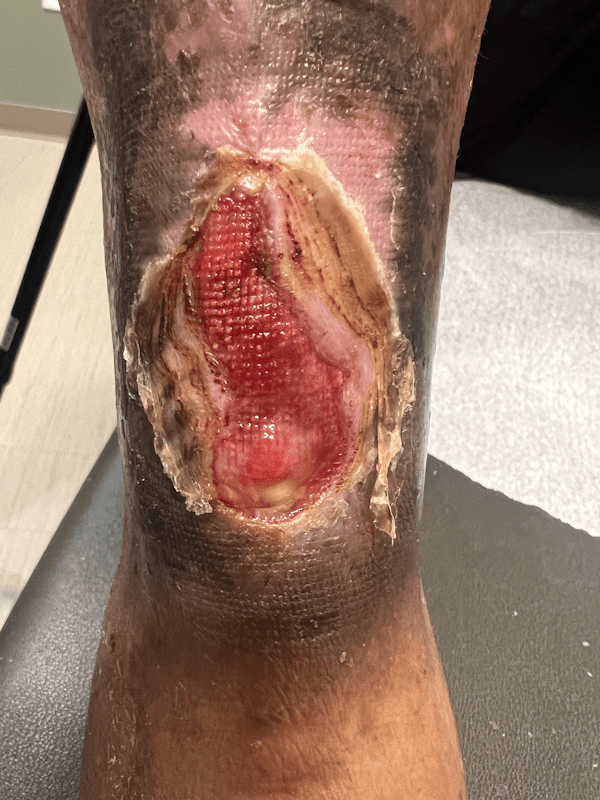
Artacent Wound® Week 10:
Wound Size: 6.0 x 2.8 x 0.1cm
Wound Area: 16.8 cm²
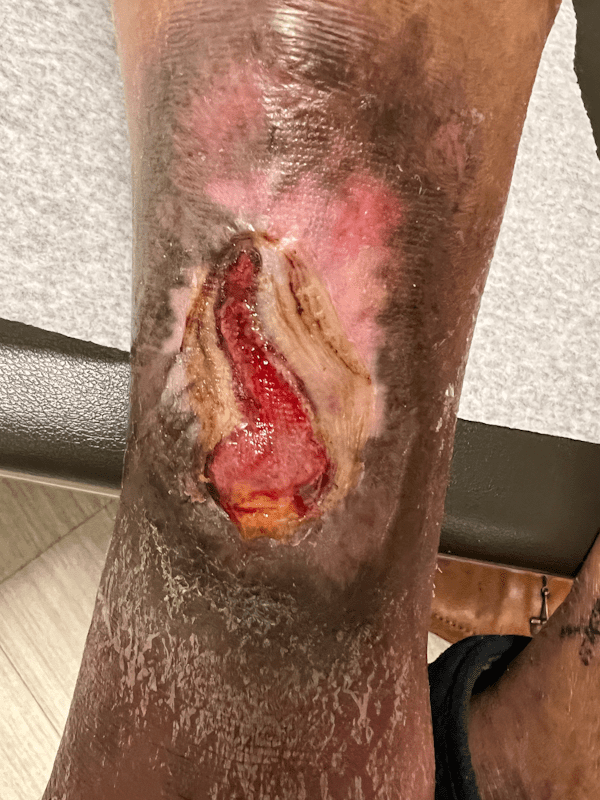
Artacent Wound® Week 11:
Wound Size: 5.6 x 2.1 x 0.05cm
Wound Area: 5.88 cm²
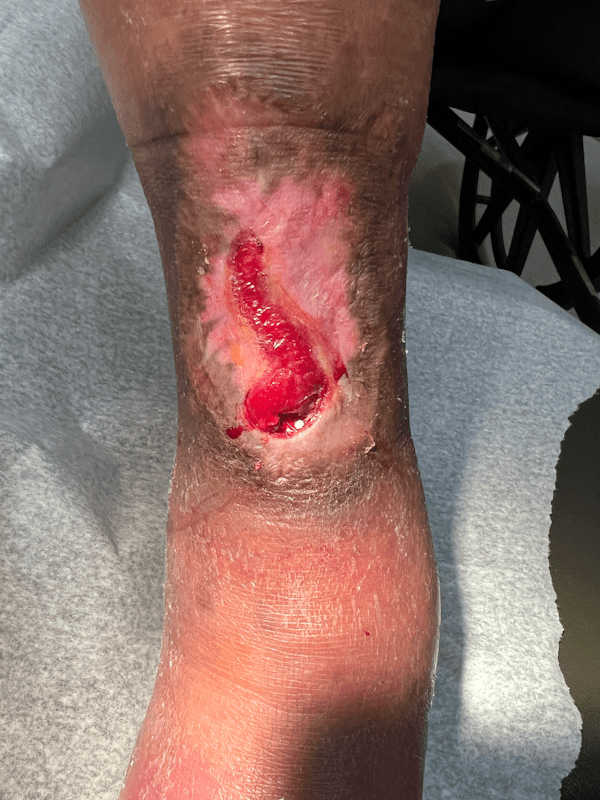
Artacent Wound® Week 12:
Wound Size: 5.3 x 2.0 x 0.05cm
Wound Area: 5.3 cm²
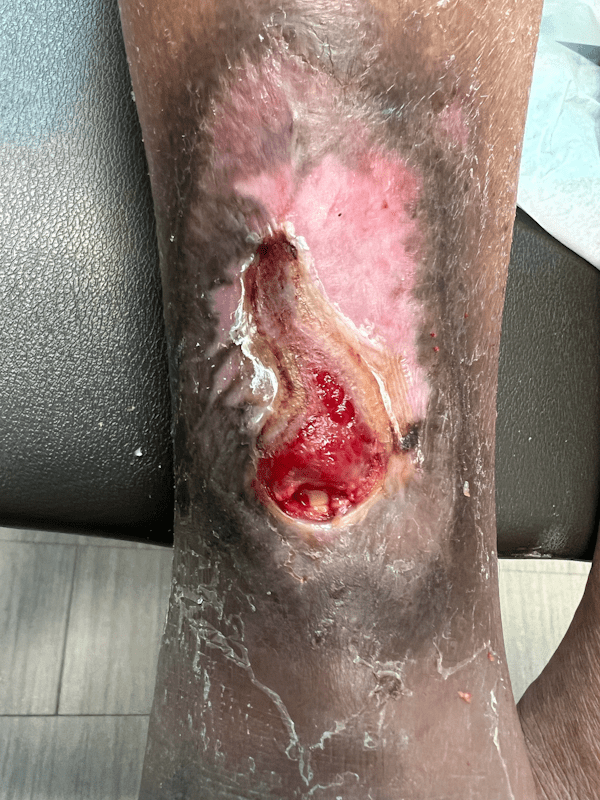
Artacent Wound® Week 13:
Wound Size: 3.2 x 1.9 x 0.05cm
Wound Area: 3.04 cm²
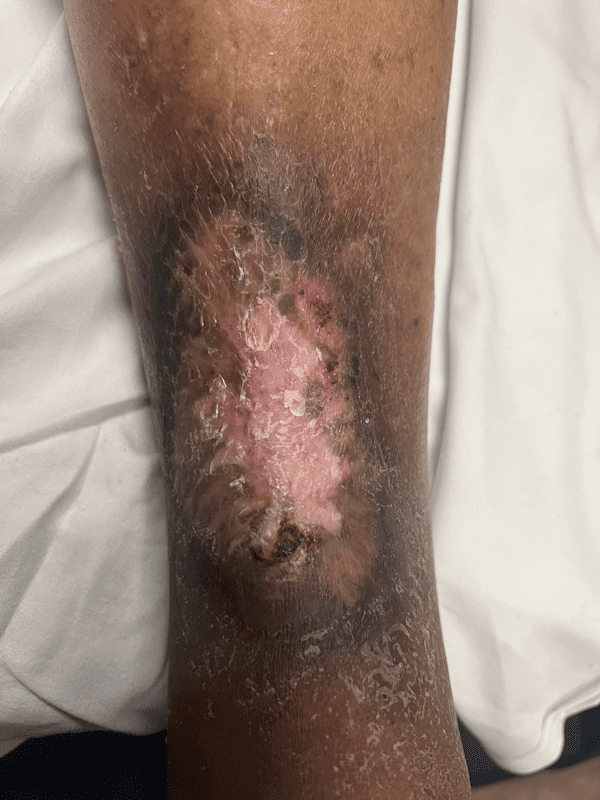
Post treatment closure
PRECAUTION: This case study presents a real-world outcome when the product was used per its indicated use, for this patient only. The appropriateness and suitability of using this product should be assessed by an independent evaluation of the patient. This case study should not be construed as making or insinuating any claims respecting clinical outcomes or any biologic / biochemical / chemical processes or modes of action.



 Marc Stemler,
Marc Stemler,  Mora Melican, Ph.D., VP of Operations, Research & Development
Mora Melican, Ph.D., VP of Operations, Research & Development LESA CATALON,
LESA CATALON, DAVID CASTILLE,
DAVID CASTILLE,




 JOSH WILLETT,
JOSH WILLETT, JEFF MONTGOMERY,
JEFF MONTGOMERY, MIKE RIDDLE,
MIKE RIDDLE, BENJAMIN KIMBALL,
BENJAMIN KIMBALL, DOUG PAYNE,
DOUG PAYNE, JOE SPELL,
CEO
JOE SPELL,
CEO